This is very good question. Many boat owners will be familiar with galvanic corrosion and the costly nature of attending to the issues associated with metals in water. All metals will corrode when exposed to oxygen and moisture and revert back to the basic natural state of their raw material.
Without preventative measures and proper maintenance, corrosion can occur very quickly and be costly. However, there are simple measures which can be taken to significantly reduce the effects of corrosion in terms of time and cost.
Metals are graded in 'The Galvanic Series of Metals', reflecting the ease at which they corrode. Softer metals corrode at a higher rate than harder metals. Sea-water is a great electrical conductor and can be a major contributor to the rate of corrosion.
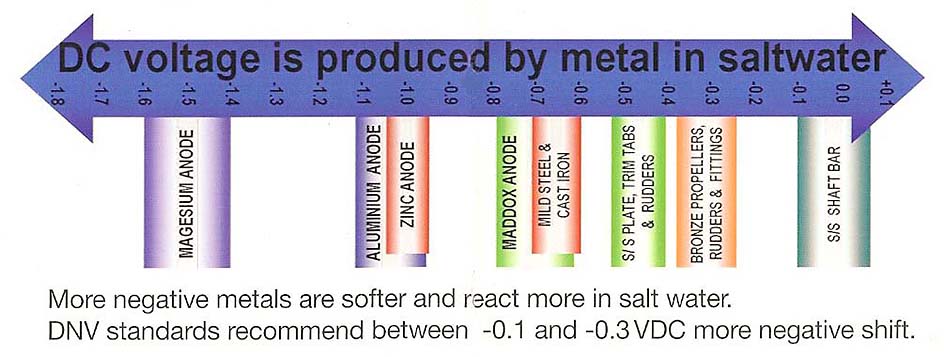
Anode selection should consider the environment, the type of water and the type of vessel.
For example, extruded anodes should be used in saltwater to protect non-metallic hull vessels, zinc anodes to protect metallic hull vessels, softer cast sacrificial anodes vessels in fresh water environments, aluminium alloy anodes near a river source and further upriver, and magnesium anodes in environments where there are numerous dissolved minerals, for example a clay water bed or fresh water.
In warm water a harder sacrificial anode is appropriate, in cold saltwater more sacrificial anode surface area is required to achieve adequate protection.
The flow rate of the water will also affect the consumption rate of sacrificial anodes. Soft cast sacrificial anodes will be consumed by water erosion and may not achieve the required protection levels for the expected lifetime. Additional anodes or a harder, extruded sacrificial anode will be required in areas of higher water flow rates.
* * *
The briefly described scenarios highlight the importance of considering the environment that the vessel resides in to achieve the greatest efficiency and performance of a sacrificial anode.

